Are you considering a future as a Medical Laboratory Technologist (MLT) in Canada? Whether you:
- Are currently studying or have completed your education in another country, or
- Already practising as an MLT outside of Canada,
this article is for you.
📘 What This Article Covers
This article guides you through the step-by-step process for internationally educated applicants to become Medical Laboratory Technologists (MLTs) in Canada. You will learn about:
- Provincial Regulation
- The CAMLPR Flexible Pathways Process
- Educational Requirements
- CAMLPR Fields-of-Practice Competency
- Final Registration Steps
Becoming an MLT in Canada is a regulated process. This means you must meet specific national standards designed to ensure safe, high-quality care for patients—regardless of your background or country of origin.
WHAT IS A MEDICAL LABORATORY TECHNOLOGIST (MLT)?
Medical laboratory technologists conduct medical laboratory tests, experiments, and analyses that help physicians diagnose, treat, monitor, and prevent disease. Their work is essential to patient care across Canada’s healthcare system.
It is important to note that medical laboratory technologists are not the same as medical laboratory assistants or other related technical roles. While some tasks may overlap, medical laboratory assistants typically focus on pre-analytical duties like collecting specimens, preparing reagents, and maintaining equipment. MLTs, on the other hand, are trained and licensed to perform more advanced analytical procedures and exercise professional judgment in their practice.
(Please click here to learn more about the medical laboratory technologist profession in Canada.)
👉 Continue reading this article if you are seeking to become a medical laboratory technologist in Canada, as it outlines the registration process you will need to follow.
UNDERSTANDING REGULATION IN CANADA
Medical laboratory technologists are regulated health professionals in most provinces across Canada. The registration process varies depending on whether the profession is regulated in the province where you plan to work or not.
The profession is currently regulated in nine provinces:
Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, Quebec, Alberta, and Saskatchewan.
It is not regulated in British Columbia, the Northwest Territories, Yukon, or Nunavut.
Which Registration Process Applies to You?
Depending on where you plan to work, you will follow one of the following two registration processes:
1. CAMLPR Flexible Pathways Process
- If you are seeking to work in Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, or Saskatchewan, you will follow the CAMLPR Flexible Pathways and must register with the regulatory body in that province.
- If you plan to work in British Columbia, Yukon, Northwest Territories, or Nunavut, you will still be required to successfully complete the CAMLPR Fields-of-Practice Competency Exams once they come into effect., although registration is not currently required in these jurisdictions.
2. Separate Provincial Process
- If you intend to work in Quebec or Alberta, you must apply directly to:
- The Ordre professionnel des technologistes médicaux du Québec (OPTMQ), or
- The College of Medical Laboratory Technologists of Alberta (CMLTA),
as these provinces are not part of the CAMLPR Flexible Pathways process.
📍 For more information, please refer to the “Where Do You Plan to Work” section on our website.
👉 Continue reading this article if you are seeking to work in Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, or Saskatchewan—where the profession is regulated and the CAMLPR Flexible Pathways process applies to your registration journey.
CAMLPR FLEXIBLE PATHWAYS IN REGULATED PROVINCES: WHAT YOU NEED TO KNOW
In provinces where the profession is regulated, each provincial regulatory body has the legislative authority to set and enforce the qualifications, standards, and competencies required to practise as a medical laboratory technologist in that province.
These regulatory bodies are member organizations of the Canadian Alliance of Medical Laboratory Professionals Regulators (CAMLPR).
While CAMLPR does not directly regulate the profession, it supports its members by promoting fair, transparent, inclusive, and consistent regulatory practices across Canada. CAMLPR also coordinates national efforts to align standards and ensure that all applicants—regardless of where they were educated—are assessed against shared expectations for knowledge, skills, and professional judgment.
This collaborative regulatory framework helps protect the public interest by ensuring that all practising MLTs in regulated jurisdictions meet rigorous professional, ethical, and technical standards.
To begin your path toward registration in a regulated province, your first step is to ensure that your education and training meet Canadian standards.
👉 Continue reading this article if you plan to practise in a regulated province and want to understand the educational requirements you must meet to become a medical laboratory technologist in Canada.
MEETING THE REQUIREMENTS FOR REGISTRATION: WHERE TO BEGIN
If you were educated or have practised as a Medical Laboratory Technologist (MLT) outside of Canada, you will follow the CAMLPR Flexible Pathways process.
Your first step is to complete a Prior Learning Assessment (PLA), which evaluates your education—and where applicable, your professional experience—to determine whether your background is comparable to a CAMLPR-approved Canadian program.
You may fall into one or more of the following categories:
- Internationally Educated Applicant
You completed a medical laboratory technology or science program outside Canada.
- Practising MLT Abroad
You are currently working as an MLT outside of Canada.
All internationally educated applicants begin by completing a Prior Learning Assessment (PLA).
- If your education is confirmed as comparable to a CAMLPR-approved Canadian program, you may proceed to write the CAMLPR Fields-of-Practice Exams as the next step in the registration process.
- If your education is not deemed comparable—or if you do not yet have the required academic background—you will need to complete a CAMLPR-approved Canadian medical laboratory technology or science program before proceeding with registration.
👉 If you are interested in becoming an MLT in Canada but have not yet started any relevant education, your first step is to complete a CAMLPR-approved Canadian medical laboratory technology/science program.
These include programs that are accredited through the Health Standards Organization’s (HSO) EQual™ Accreditation program. Approved programs may have full accreditation status, are accredited with conditions, or are listed as admitted programs working toward full accreditation with EQual™.
👉 Continue reading this article if you are an internationally educated applicant or an MLT practising outside of Canada—you may be eligible to proceed through the CAMLPR Flexible Pathways process.
STEPS IN THE CAMLPR FLEXIBLE PATHWAYS PROCESS
If you are an internationally educated applicant or an MLT practising outside of Canada planning to work in a regulated province that follows the CAMLPR Flexible Pathways, this section is for you.
The Flexible Pathways Process is designed to assess your education, competencies, and readiness to practise as a Medical Laboratory Technologist (MLT) in Canada. It ensures that all applicants are evaluated against national standards for safe and effective practice.
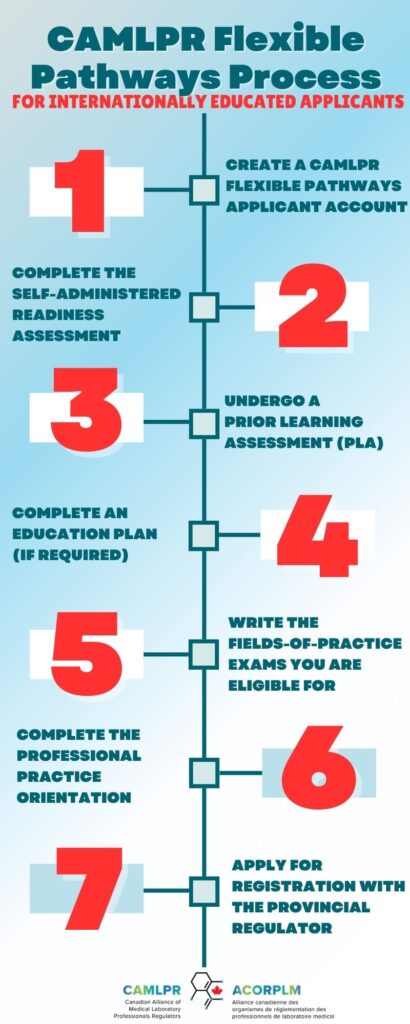
Here are the key steps in the process:
- Create a Flexible Pathways applicant account
Begin by creating your applicant account through CAMLPR’s Online Portal. This is where you will submit documentation, track your progress, and access required materials throughout the process.
Create a CAMLPR Flexible Pathways account if you:
- Want to work as a medical laboratory technologist in Canada,
- Have completed an international MLT program or a program in a related medical or health science field, and
- Plan to work in a province or territory that requires the CAMLPR Flexible Pathways process.
- Complete the Self-Administered Readiness Assessment for the fields of practice you intend to work in
The self-administered readiness assessment is designed to help applicants evaluate if they are ready to write the CAMLPR Fields-of-Practice Exams and identify areas in which they may need additional preparation.
The readiness assessment contains sample examination questions in each field of practice to help you:
- Become familiar with the question format of the competency exams
- Assess your strengths and development needs according to Canadian professional standards
- Help focus your preparation on specific competency areas where additional study may be beneficial
Once you have your Flexible Pathways account creation and fee payment approved by CAMLPR, you will be able to complete the self-administered readiness assessment in the Flexible Pathways application portal.
- Undergo a Prior Learning Assessment (PLA) for those fields of practice
The Prior Learning Assessment is a formal evaluation of your education, clinical training, work experience, and language proficiency to determine if they meet Canadian standards for Medical Laboratory Technologists. This comprehensive assessment reviews both theoretical coursework and hands-on laboratory experience to ensure your academic preparation is current and sufficient.
All PLA applicants must also demonstrate language proficiency in English or French, as outlined in CAMLPR’s Language Policy (I-03).
To complete the PLA, you must submit documentation related to your education, work experience, and language proficiency. Most documents must be sent directly to CAMLPR by the issuing institutions or organizations.
➡️ See the Summary of Required Documents for details.
🕒 Estimated Timelines for the PLA Process
The Prior Learning Assessmentprocess can take several weeks to several months to complete, depending on your individual circumstances. Timelines may vary based on:
- Processing times at your previous schools, clinical sites, or employers
- Delays in receiving official documents such as transcripts or reference letters
- Credential evaluations and language test reporting times
- Completion of CAMLPR’s internal assessment once all documents are received
👉 Tip: Begin collecting your documents as early as possible and contact your institutions directly to understand their processing timelines. CAMLPR will notify you once your PLA file is complete and under review.
- Complete an Education Plan if required as a result of your PLA
If gaps are identified in an applicant’s field-of-practice competencies, CAMLPR requires the applicant to complete an Education Plan before writing the corresponding field-of-practice exam. This educational support helps ensure practitioners meet Canadian practice standards before entering the profession.
- Write the Fields-of-Practice exams you are eligible for
Once you are deemed eligible, you will be invited to write the relevant national CAMLPR Fields-of-Practice Competency Exams to confirm your readiness for practise in Canada. These exams evaluate entry-level competencies outlined in the CAMLPR Competency Profiles, support registration in one or multiple fields, and ensure a fair, consistent process for all applicants. They also help address the shortage of medical laboratory technologists in Canada by streamlining access for qualified professionals.
👉 Click here to get information about Exam Dates, registration, and scheduling.
- Complete the Professional Practice Orientation
Before applying for registration, you must complete a mandatory orientation to learn about Canadian professional practice expectations, ethics, and regulatory responsibilities. The Professional Practice Orientation is a required, self-paced online course that introduces medical laboratory technologists to the ethical, legal, and professional standards they are expected to meet in Canada.
Once you have successfully completed the CAMLPR Flexible Pathways Process, your next step is to apply for registration with the relevant provincial regulatory body. In most provinces, medical laboratory technologists must be registered before they can begin working. Only registered individuals may:
- Legally practise medical laboratory technology
- Use the protected titles “MLT” or “Medical Laboratory Technologist”
Registration confirms that you meet the required standards and are qualified to provide safe, ethical care.
Registration Requirements and Timelines
Each province has its own requirements and timelines, but generally, you will need to:
- Submit an application to the relevant provincial regulator
- Provide any additional documents they request
- Renew your registration annually, including proof of ongoing competence
“Fit and Proper Person” Requirement
To be registered as an MLT in Canada, you must be considered a “fit and proper person,” ensuring you are qualified, ethical, and safe to practise.
👉 Click here to learn more about “Fit and Proper Person” Requirements for Medical Laboratory Technologists in Canada.
🗓️ IMPORTANT: New Application Process Effective November 1, 2025
As of this date, all internationally educated MLTs and those with non-traditional education must write the CAMLPR Field-of-Practice Exams.
The CAMLPR Application Portal is now open. If you want to write the September 2025 or November 2025 exams, you should begin your application and PLA process now.
If you have previously engaged with CSMLS or received a Statement of Eligibility, please review the guidance below to understand how the new process may apply to you:
Click here to learn how the CAMLPR process applies if you:
- Have already passed a CSMLS exam
- Attempted but did not pass a CSMLS exam
- Received a CSMLS Statement of Eligibility (but haven’t written the exam yet)
- Are a CSMLS PLA client and have received a Statement of Eligibility
⚠️Work Eligibility Disclaimer
Please note: Completing the CAMLPR Flexible Pathways process and passing the required assessments does not guarantee employment. You must be registered with a provincial regulatory body before legally working as a Medical Laboratory Technologist (MLT) in a regulated province.
⚠️Immigration Disclaimer
CAMLPR does not provide immigration advice or services. If you are not a Canadian citizen or permanent resident, you may need a valid visa, study permit, or work permit to enter or remain in Canada. For details, visit Immigration, Refugees and Citizenship Canada (IRCC).
📬 Need Help? We are Here for You
Have questions about your eligibility, the application process, or required documents?
CAMLPR is here to support you at every step of your journey.
For assistance:
📧 Email : applicants@camlpr.org
🌐 Visit : www.camlpr.org
We encourage you to review the resources on our website and reach out if you need any guidance.

